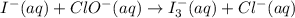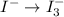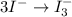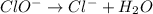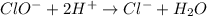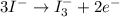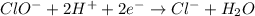Chemistry, 15.11.2019 19:31 truelove9288
Using the half reaction method balance to the redox equation in acidic solution: i-(aq) + clo-(aq) → i3-(aq) + cl-(aq) indicate the correct equation below. view available hint(s) using the half reaction method balance to the redox equation in acidic solution: i-(aq) + clo-(aq) → i3-(aq) + cl-(aq) indicate the correct equation below. 3i-(aq)+2h+(aq)+clo-(aq)→i3-(aq)+cl -(aq)+h2o(l) 3i-(aq)+2h+(aq)+clo-(aq)→i3-(aq)+cl -(aq)+h2o(l)+2e- i-(aq)+10h+(aq)+clo-(aq)→i3-(aq)+cl -(aq)+10h2o(l) i-(aq)+2h+(aq)+clo-(aq)→6i3-(aq)+cl -(aq)+h2o(l)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2
You know the right answer?
Using the half reaction method balance to the redox equation in acidic solution: i-(aq) + clo-(aq)...
Questions






Mathematics, 05.04.2021 23:40

Arts, 05.04.2021 23:40


Computers and Technology, 05.04.2021 23:40

Mathematics, 05.04.2021 23:40




History, 05.04.2021 23:40


Computers and Technology, 05.04.2021 23:40




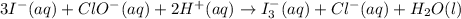
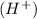 at that side where the less number of hydrogen are present.
at that side where the less number of hydrogen are present.