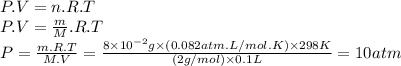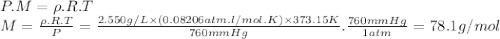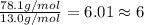Chemistry, 15.11.2019 18:31 savidgarcia303
Part a. at present, automobile batteries are sealed. when lead storage batteries discharge, they produce hydrogen. suppose the void volume in the battery is 100 ml at 1 atm of pressure and 25°c. what would be the pressure increase if 8×10−2 g h2 were produced by the discharge of the battery? atm
part b. a certain compound containing only carbon and hydrogen was found to have a vapor density of 2.550 g/l at 100°c and 760 mm hg. if the empirical formula of this compound is ch, what is the molecular formula of this compound?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1


Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1

Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2
You know the right answer?
Part a. at present, automobile batteries are sealed. when lead storage batteries discharge, they pro...
Questions

Chemistry, 09.09.2021 02:50


Mathematics, 09.09.2021 02:50


Law, 09.09.2021 02:50

Mathematics, 09.09.2021 02:50

Mathematics, 09.09.2021 02:50


Physics, 09.09.2021 02:50

Mathematics, 09.09.2021 02:50

Mathematics, 09.09.2021 02:50


Mathematics, 09.09.2021 02:50

History, 09.09.2021 02:50





Mathematics, 09.09.2021 02:50

History, 09.09.2021 02:50