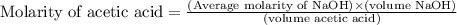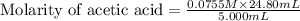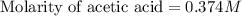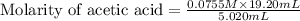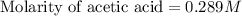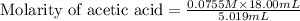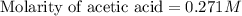Chemistry, 15.11.2019 18:31 beckers0115
Calculate and enter the molarity of your three acetic acid trials using the volume of standardized naoh solution required for each and the average molarity of the naoh solution from the standardization trials with khp. you should report 3 significant figures, e. g. 0.488 m.
entry # vol acetic acid(ml) vol na0h(ml) m acetic acid
#1: 5.000 24.80
#2: 5.020 19.20
#3: 5.019 18.00

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
You know the right answer?
Calculate and enter the molarity of your three acetic acid trials using the volume of standardized n...
Questions




Mathematics, 16.11.2020 14:00


Mathematics, 16.11.2020 14:00

Mathematics, 16.11.2020 14:00


History, 16.11.2020 14:00

Mathematics, 16.11.2020 14:00

Biology, 16.11.2020 14:00

English, 16.11.2020 14:00

Mathematics, 16.11.2020 14:00

Mathematics, 16.11.2020 14:00


Mathematics, 16.11.2020 14:00


Biology, 16.11.2020 14:00

Biology, 16.11.2020 14:00

Mathematics, 16.11.2020 14:00