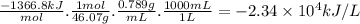Chemistry, 15.11.2019 17:31 jessicachichelnitsky
Ethanol (c2h5oh) is currently blended with gasoline as an automobile fuel. calculate the heat produced per liter of ethanol by combustion of ethanol under constant pressure. ethanol has a density of 0.789 g/ml.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2



You know the right answer?
Ethanol (c2h5oh) is currently blended with gasoline as an automobile fuel. calculate the heat produc...
Questions

History, 05.01.2021 19:40



Geography, 05.01.2021 19:40

Mathematics, 05.01.2021 19:40





History, 05.01.2021 19:40


Mathematics, 05.01.2021 19:40

Mathematics, 05.01.2021 19:40




History, 05.01.2021 19:40

Health, 05.01.2021 19:50