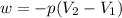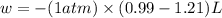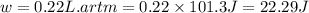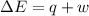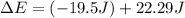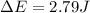Chemistry, 15.11.2019 17:31 lovemusic4
Ahelium-filled balloon at 310.0 k and 1 atm, contains 0.05 g he, and has a volume of 1.21 l. it is placed in a freezer (t = 235.0 k), and its volume decreases to 0.99 l. find δe for the gas (in joules). (cp of he = 20.8 j/mol k.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
You know the right answer?
Ahelium-filled balloon at 310.0 k and 1 atm, contains 0.05 g he, and has a volume of 1.21 l. it is p...
Questions

Mathematics, 09.09.2021 06:30

History, 09.09.2021 06:30

Mathematics, 09.09.2021 06:30

Mathematics, 09.09.2021 06:30


Biology, 09.09.2021 06:30



Mathematics, 09.09.2021 06:30


Health, 09.09.2021 06:30




Chemistry, 09.09.2021 06:30

Mathematics, 09.09.2021 06:30


Social Studies, 09.09.2021 06:30

Mathematics, 09.09.2021 06:30

Social Studies, 09.09.2021 06:30

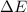 of the gas is 2.79 Joules.
of the gas is 2.79 Joules.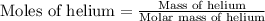
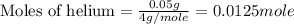
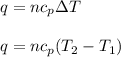
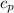 = specific heat of helium = 20.8 J/mol.K
= specific heat of helium = 20.8 J/mol.K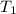 = initial temperature = 310.0 K
= initial temperature = 310.0 K = final temperature = 235.0 K
= final temperature = 235.0 K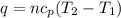
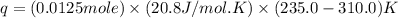
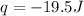
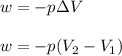
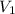 = initial volume = 1.21 L
= initial volume = 1.21 L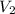 = final volume = 0.99 L
= final volume = 0.99 L