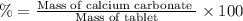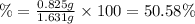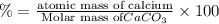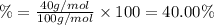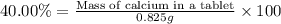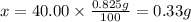Chemistry, 15.11.2019 07:31 harmonyfern5648
(a tablet containing calcium carbonate and fillers with a mass of 1.631 g was dissolved in hcl. after the fillers were filtered out, the hcl was neutralized by adding sodium carbonate. the resulting precipitate was pure calcium carbnonate (with the fillers removed). the solid calcium carbonate was collected on a watch glass that had a mass of 46.719 g when empty. after teh calcium carbonate had been allowed to dry, the mass of the watch glass plus product was found to be 47.544 g.
1. what is the mass of pure calcium carbonate product collected at the end of the experiment?
2. calculate the mass % calium carbonate in the tablet.
3. calculate the mass percent calcium in calcium carbonate. this calculation is theoretical and is independent of the data provided.
4. calculate the number of grams of calcium that were in the tablet.
(hint: this can be obtained by using the answers to question 1 and 3)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
You know the right answer?
(a tablet containing calcium carbonate and fillers with a mass of 1.631 g was dissolved in hcl. afte...
Questions



Chemistry, 27.01.2020 12:31

Social Studies, 27.01.2020 12:31






Mathematics, 27.01.2020 12:31

Mathematics, 27.01.2020 12:31








Chemistry, 27.01.2020 12:31