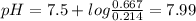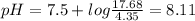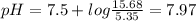Chemistry, 15.11.2019 06:31 cakecake15
A50.00-ml sample of bleach solution contains 0.214 m hclo and 0.667 m naclo. the ka of hypochlorous acid is 3.0 ✕ 10−8. find the ph of the solution. the solution is then divided in half. a) to one half of the original solution, 10.00 ml of 0.100 m naoh is added. what is the final ph of this solution? b) to the other half of the original solution, 1.00 ml of 0.100 m hcl is added. what is the final ph of this solution

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3


You know the right answer?
A50.00-ml sample of bleach solution contains 0.214 m hclo and 0.667 m naclo. the ka of hypochlorous...
Questions

English, 12.07.2019 13:00

Mathematics, 12.07.2019 13:00

Chemistry, 12.07.2019 13:00

Mathematics, 12.07.2019 13:00


History, 12.07.2019 13:00

Mathematics, 12.07.2019 13:00



Mathematics, 12.07.2019 13:00

Mathematics, 12.07.2019 13:00

Mathematics, 12.07.2019 13:00

Mathematics, 12.07.2019 13:00


Geography, 12.07.2019 13:00



Mathematics, 12.07.2019 13:00

Social Studies, 12.07.2019 13:00

![pH=pKa+log\frac{[salt]}{[acid]}](/tpl/images/0375/5235/ec35f.png)