
Nh4no3, whose heat of solution is 25.7 kj/mol, is one substance that can be used in cold pack. if the goal is to decrease the temperature from 25.0 °c to 5.0 °c, how many grams of nh4no3 should we use for every 100.0 g of water in the cold pack? assume no heat was lost outside of cold pack, and the specific heat of the resulted solution was the same as water, or 4.184 j/(g•°c).

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
You know the right answer?
Nh4no3, whose heat of solution is 25.7 kj/mol, is one substance that can be used in cold pack. if th...
Questions

Mathematics, 15.01.2021 23:50


Mathematics, 15.01.2021 23:50

English, 15.01.2021 23:50



Mathematics, 15.01.2021 23:50

Mathematics, 15.01.2021 23:50

Mathematics, 15.01.2021 23:50




Mathematics, 15.01.2021 23:50


English, 15.01.2021 23:50

English, 15.01.2021 23:50

Mathematics, 15.01.2021 23:50

Law, 15.01.2021 23:50

Mathematics, 15.01.2021 23:50

Health, 15.01.2021 23:50

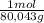 X
X

