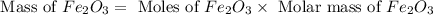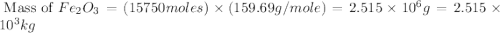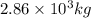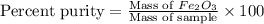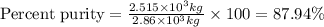One of the reactions that occurs in a blast furnace, in which iron ore is converted to cast iron, is fe2o3 + 3co → 2fe + 3co2 suppose that 1.79 × 103 kg of fe is obtained from a 2.86 × 103 kg sample of fe2o3. assuming that the reaction goes to completion, what is the percent purity of fe2o3 in the original sample?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

You know the right answer?
One of the reactions that occurs in a blast furnace, in which iron ore is converted to cast iron, is...
Questions

Mathematics, 12.03.2021 23:00

Mathematics, 12.03.2021 23:00


Mathematics, 12.03.2021 23:00

Mathematics, 12.03.2021 23:00


Mathematics, 12.03.2021 23:00

Mathematics, 12.03.2021 23:00

Mathematics, 12.03.2021 23:00

Mathematics, 12.03.2021 23:00

Mathematics, 12.03.2021 23:00








Mathematics, 12.03.2021 23:00

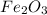 in the original sample is 87.94 %
in the original sample is 87.94 %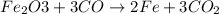
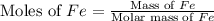
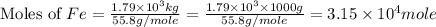
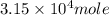 of Fe produced from
of Fe produced from 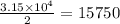 mole of
mole of