Chemistry, 14.11.2019 20:31 unknown54321
The value of δ°′δg°′ for the conversion of glucose-6-phosphate to fructose-6-phosphate (f6p) is +1.67 kj/mol+1.67 kj/mol . if the concentration of glucose-6-phosphate at equilibrium is 1.85 mm1.85 mm , what is the concentration of fructose-6-phosphate? assume a temperature of 25.0°c25.0°c .

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Temperature and kinetic energy are proportional. a) adirectly b) directly c) indirectly
Answers: 2

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 23.06.2019 04:40
Equal numbers of moles of he(g), ar(g), and ne(g) are placed in a glass vessel at room temperature. if the vessel has a pinhole-sized leak, which of the following will be true regarding the relative values of the partial pressures of the gases remaining in the vessel after some of the gas mixture has effused?
Answers: 1

Chemistry, 23.06.2019 07:30
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes no correct anwser get brainliest
Answers: 1
You know the right answer?
The value of δ°′δg°′ for the conversion of glucose-6-phosphate to fructose-6-phosphate (f6p) is +1.6...
Questions


Computers and Technology, 31.08.2019 03:10

Computers and Technology, 31.08.2019 03:10

Mathematics, 31.08.2019 03:10


Mathematics, 31.08.2019 03:10

Computers and Technology, 31.08.2019 03:10

World Languages, 31.08.2019 03:10




Mathematics, 31.08.2019 03:10

Biology, 31.08.2019 03:10

History, 31.08.2019 03:10



Mathematics, 31.08.2019 03:10

Mathematics, 31.08.2019 03:10


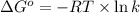
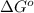 = standard Gibbs free energy = +1.67 kJ/mol = +1670 J/mol
= standard Gibbs free energy = +1.67 kJ/mol = +1670 J/mol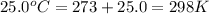
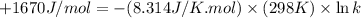
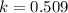
![K=\frac{[\text{fructose-6-phosphate}]}{[\text{glucose-6-phosphate}]}](/tpl/images/0374/4842/feaba.png)
![0.509=\frac{[\text{fructose-6-phosphate}]}{1.85mM}](/tpl/images/0374/4842/d0c84.png)