

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
What is the hybridization of the central atom in each of the following? 1. beryllium chloride 2. ni...
Questions





Mathematics, 11.10.2020 16:01

Mathematics, 11.10.2020 16:01


Mathematics, 11.10.2020 16:01

English, 11.10.2020 16:01

Mathematics, 11.10.2020 16:01


Chemistry, 11.10.2020 16:01


Geography, 11.10.2020 16:01

History, 11.10.2020 16:01

Mathematics, 11.10.2020 16:01



Mathematics, 11.10.2020 16:01

Mathematics, 11.10.2020 16:01

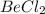 is,
is, 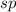
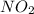 is,
is, 
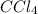 is,
is, 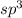
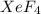 is,
is, 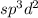
![\text{Number of electron pair}=\frac{1}{2}[V+N-C+A]](/tpl/images/0374/5204/9e987.png)
![\text{Number of electrons}=\frac{1}{2}\times [2+2]=2](/tpl/images/0374/5204/7d797.png)
![\text{Number of electrons}=\frac{1}{2}\times [4]=2](/tpl/images/0374/5204/34bd3.png)
![\text{Number of electrons}=\frac{1}{2}\times [4+4]=4](/tpl/images/0374/5204/b4293.png)
![\text{Number of electrons}=\frac{1}{2}\times [8+4]=6](/tpl/images/0374/5204/47c28.png)


