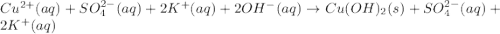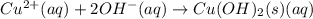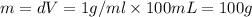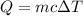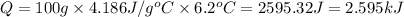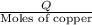Consider two solutions, the first being 50.0 ml of 1.00 m cuso4 and the second 50.0 ml of 2.00 m koh. when the two solutions are mixed in a constant-pressure calorimeter, a precipitate forms and the temperature of the mixture rises from 21.5 ∘c to 27.7 ∘c.
a) before mixing, how many grams of cu are present in the solution of cuso4?
b) predict the identity of the precipitate in the reaction.
c) write complete equation for the reaction that occurs when the two solutions are mixed.
d) write net ionic equation for the reaction that occurs when the two solutions are mixed.
e) from the calorimetric data, calculate δh for the reaction that occurs on mixing. assume that the calorimeter absorbs only a negligible quantity of heat, that the total volume of the solution is 100.0 ml, and that the specific heat and density of the solution after mixing are the same as that of pure water.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3
You know the right answer?
Consider two solutions, the first being 50.0 ml of 1.00 m cuso4 and the second 50.0 ml of 2.00 m koh...
Questions









Biology, 26.02.2022 21:10

Social Studies, 26.02.2022 21:10

Chemistry, 26.02.2022 21:10









Mathematics, 26.02.2022 21:20


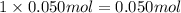 of copper
of copper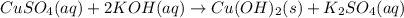
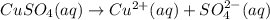 ..[1]
..[1]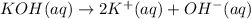 ..[2]
..[2]