
Chemistry, 23.08.2019 20:40 hbkakabryce0p3fkoq
Use electron transfer or electron shift to identify what is oxidized and what is reduced in each reaction :
a) 2na(s) + br2(l) > 2nabr(s)
b) h2(g) + cl2(g) > 2hcl(g)
c) 2li(s) + f2(g) > 2lif(s)
d) s(s) + cl2(g) > scl2(g)
e)n2(g) + 2o2(g) > 2no2(g)
f) mg(s) +cu(no3)2(aq) = mg(no3)2(aq) + cu(s)
for each reaction above, identify the reducing agent and the oxidizing agent

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1
You know the right answer?
Use electron transfer or electron shift to identify what is oxidized and what is reduced in each rea...
Questions


Arts, 06.04.2021 07:00

Business, 06.04.2021 07:00

Mathematics, 06.04.2021 07:00



Geography, 06.04.2021 07:00



Mathematics, 06.04.2021 07:00

Mathematics, 06.04.2021 07:00



English, 06.04.2021 07:00

Physics, 06.04.2021 07:00


Biology, 06.04.2021 07:00

Mathematics, 06.04.2021 07:00

History, 06.04.2021 07:00

Mathematics, 06.04.2021 07:00

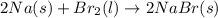
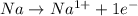
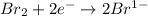
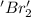 is reduced in this reaction. The reducing agent is, 'Na' and oxidizing agent is,
is reduced in this reaction. The reducing agent is, 'Na' and oxidizing agent is, 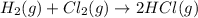
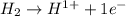

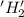 is oxidized and
is oxidized and 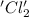 is reduced in this reaction. The reducing agent is,
is reduced in this reaction. The reducing agent is, 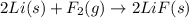
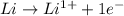
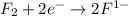
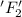 is reduced in this reaction. The reducing agent is, 'Li' and oxidizing agent is,
is reduced in this reaction. The reducing agent is, 'Li' and oxidizing agent is, 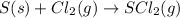
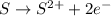
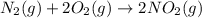
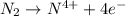

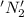 is oxidized and
is oxidized and 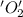 is reduced in this reaction. The reducing agent is,
is reduced in this reaction. The reducing agent is, 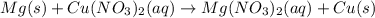
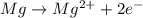
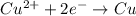
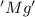 is oxidized and
is oxidized and 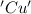 is reduced in this reaction. The reducing agent is, 'Mg' and oxidizing agent is, 'Cu'.
is reduced in this reaction. The reducing agent is, 'Mg' and oxidizing agent is, 'Cu'.

