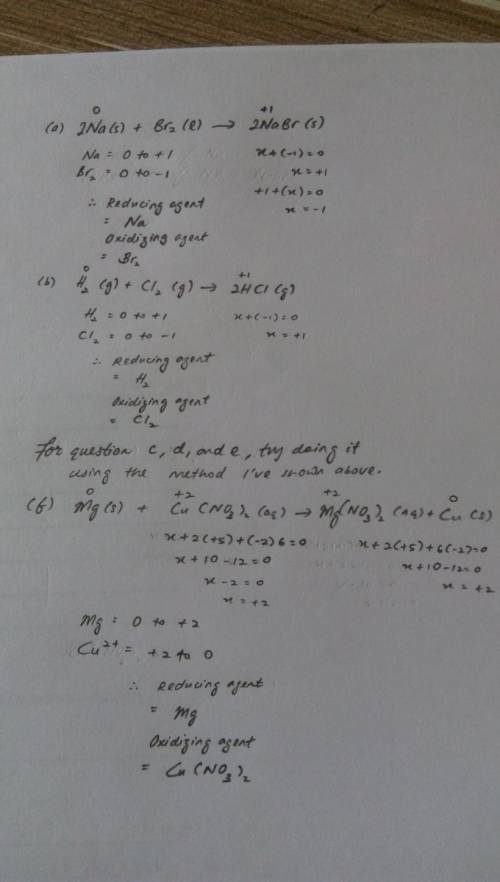Use electron transfer or electron shift to identify what is oxidized and what is reduced in each reaction :
a) 2na(s) + br2(l) > 2nabr(s)
b) h2(g) + cl2(g) > 2hcl(g)
c) 2li(s) + f2(g) > 2lif(s)
d) s(s) + cl2(g) > scl2(g)
e)n2(g) + 2o2(g) > 2no2(g)
f) mg(s) +cu(no3)2(aq) = mg(no3)2(aq) + cu(s)
for each reaction above, identify the reducing agent and the oxidizing agent

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Can you reduce your impairment by drinking coffee or cold showers? true or false?
Answers: 3

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
You know the right answer?
Use electron transfer or electron shift to identify what is oxidized and what is reduced in each rea...
Questions


Mathematics, 20.08.2019 05:30



Health, 20.08.2019 05:30


Chemistry, 20.08.2019 05:30


Social Studies, 20.08.2019 05:30

Mathematics, 20.08.2019 05:30

Social Studies, 20.08.2019 05:30



Social Studies, 20.08.2019 05:30

Biology, 20.08.2019 05:30


Mathematics, 20.08.2019 05:30