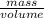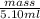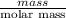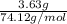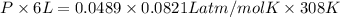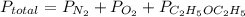Asample of 5.10 ml of diethylether (c2h5oc2h5; density = 0.7134 g/ml) is introduced into a 6.00 -l vessel that already contains a mixture of n2 and o2, whose partial pressures are pn2 = 0.752 atm and po2 = 0.206 atm. the temperature is held at 35.0 °c, and the diethylether totally evaporates.
a) calculate the partial pressure of the diethylether.
b) calculate the total pressure in the container.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Embryos of different species look very similar, which shows that the organisms share a ancestor.
Answers: 1

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
You know the right answer?
Asample of 5.10 ml of diethylether (c2h5oc2h5; density = 0.7134 g/ml) is introduced into a 6.00 -l...
Questions





History, 17.10.2019 19:20

English, 17.10.2019 19:20




English, 17.10.2019 19:30

Mathematics, 17.10.2019 19:30

Biology, 17.10.2019 19:30


Biology, 17.10.2019 19:30



History, 17.10.2019 19:30

English, 17.10.2019 19:30


Mathematics, 17.10.2019 19:30

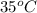 = (35 + 273) K = 308 K
= (35 + 273) K = 308 K