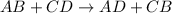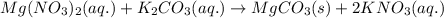Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

You know the right answer?
When an aqueous solution of magnesium nitrate is mixed with an aqueous solution of potassium carbona...
Questions


History, 13.05.2021 06:40






Health, 13.05.2021 06:40



Chemistry, 13.05.2021 06:40

History, 13.05.2021 06:40


Mathematics, 13.05.2021 06:40


Mathematics, 13.05.2021 06:40


Mathematics, 13.05.2021 06:40

Mathematics, 13.05.2021 06:40