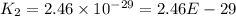Chemistry, 14.11.2019 06:31 strawberrymochi390
The reaction 2no2 → 2no + o2 obeys the rate law: rate = 1.4 x 10-2[no2]2 at 500 k . what would be the rate constant at 119 k if the activation energy is 80. kj/mol? this is a second order reaction, giving k the units of m-1s-1 this will not change with the change in temperature. do not include units in your answer. exponential numbers need to be entered like this: 2 e-1 means 2 x 10-1. the rate constant, k, at 119 k equals:

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3


Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1
You know the right answer?
The reaction 2no2 → 2no + o2 obeys the rate law: rate = 1.4 x 10-2[no2]2 at 500 k . what would be t...
Questions


French, 05.11.2020 04:00

Mathematics, 05.11.2020 04:00


Mathematics, 05.11.2020 04:00




World Languages, 05.11.2020 04:00

English, 05.11.2020 04:00

Health, 05.11.2020 04:00


Medicine, 05.11.2020 04:00

English, 05.11.2020 04:00




Biology, 05.11.2020 04:00

Business, 05.11.2020 04:00

Mathematics, 05.11.2020 04:00

![Rate=1.4\times 10^{-2}[NO_2]^2](/tpl/images/0373/7605/5818c.png) ..........(1)
..........(1)![Rate=k[NO_2]^2](/tpl/images/0373/7605/d48ef.png) ............(2)
............(2)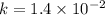
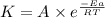
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0373/7605/6d953.png)
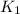 = rate constant at
= rate constant at 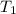 =
= 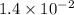
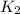 = rate constant at
= rate constant at 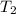 = ?
= ?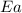 = activation energy for the reaction = 80.0 kJ/mole = 80000 J/mole
= activation energy for the reaction = 80.0 kJ/mole = 80000 J/mole![\log (\frac{K_2}{1.4\times 10^{-2}})=\frac{80000J/mole}{2.303\times 8.314J/mole.K}[\frac{1}{500}-\frac{1}{119}]](/tpl/images/0373/7605/44fe7.png)