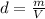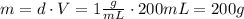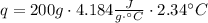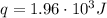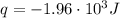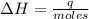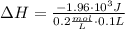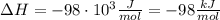Chemistry, 14.11.2019 06:31 lexybellx3
A1.00 x 102ml sample of 0.200 m aqueous hydrochloric acid is added to 1.00 x 102ml of 0.200 m aqueous ammonia in a constant-pressure calorimeter of negligible heat capacity. the following reaction occurs when the two solutionsare mixedhcl(aq)+ nh3(> nh4cl(aq)the temperature increase is 2.34°c. calculate heat change of the reaction per mole of hcl reacted. assume that the densities and specific heats of the solutions are the same as for water (1.00 g/ml and 4.184 j/g · °c, respectively)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2
You know the right answer?
A1.00 x 102ml sample of 0.200 m aqueous hydrochloric acid is added to 1.00 x 102ml of 0.200 m aqueou...
Questions

Business, 24.06.2019 17:30

Mathematics, 24.06.2019 17:30



Mathematics, 24.06.2019 17:30



English, 24.06.2019 17:30

Mathematics, 24.06.2019 17:30



History, 24.06.2019 17:30



Social Studies, 24.06.2019 17:30



Spanish, 24.06.2019 17:30


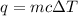 (1)
(1)