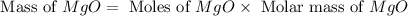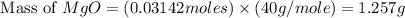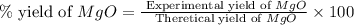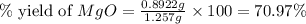Chemistry, 14.11.2019 06:31 yukichaniscool8
1. suppose 0.7542 g of magnesium reacts with excess oxygen to form magnesium oxide as the only product, what would be the theoretical yield of the product?
2. if 0.8922 g of magnesium oxide is obtained from the reaction indicated in #1 above, what would be the percent yield of the magnesium oxide?
3. suppose the percent yield was calculated to be over 100%, what possible reasons could you give to account for it?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1
You know the right answer?
1. suppose 0.7542 g of magnesium reacts with excess oxygen to form magnesium oxide as the only produ...
Questions

Mathematics, 07.04.2021 02:30

Mathematics, 07.04.2021 02:30

Mathematics, 07.04.2021 02:30

Advanced Placement (AP), 07.04.2021 02:30



Mathematics, 07.04.2021 02:30

Spanish, 07.04.2021 02:30


Biology, 07.04.2021 02:30



English, 07.04.2021 02:30

Mathematics, 07.04.2021 02:30


History, 07.04.2021 02:30

Chemistry, 07.04.2021 02:30

English, 07.04.2021 02:30


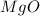 is, 1.257 grams.
is, 1.257 grams.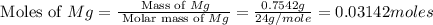
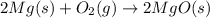
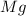 react to give 2 mole of
react to give 2 mole of