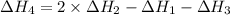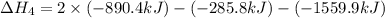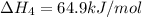1) the heat of combustion for the gases hydrogen, methane and ethane are −285.8, −890.4 and −1559.9 kj/mol respectively at 298k.
equation 1 h2 + 1⁄2o2 > h2o δh = −285.8 kj
equation 2 ch4 + 2o2 > co2 + 2h2o δh = −890.4 kj
equation 3 c2h6 + 7⁄2o2 > 2co2 + 3h2o δh = −1559.9 kj
use the above equations to calculate (at the same temperature) the heat of reaction for the following reaction:
2ch4(g) > c2h6(g) + h2(g)
solution:

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1


You know the right answer?
1) the heat of combustion for the gases hydrogen, methane and ethane are −285.8, −890.4 and −1559.9...
Questions


History, 19.10.2019 15:00



Mathematics, 19.10.2019 15:00

History, 19.10.2019 15:00


Computers and Technology, 19.10.2019 15:00


English, 19.10.2019 15:00


Mathematics, 19.10.2019 15:00


Mathematics, 19.10.2019 15:00

Biology, 19.10.2019 15:00

Mathematics, 19.10.2019 15:00

Health, 19.10.2019 15:00

Chemistry, 19.10.2019 15:00


Mathematics, 19.10.2019 15:00

 ..[1]
..[1]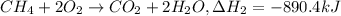 ..[2]
..[2]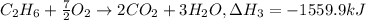 ..[3]
..[3]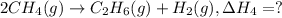 ..[4]
..[4]