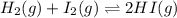H2 and i2 react in an exothermic reaction according to the following equation:
h2(g) + i2(g) double arrow yields 2hi (g)
h2 and i2 are placed in a sealed container and are allowed to reach equilibrium. what could you change about the system that would shift its equilibrium position?
a. add a catalyst.
b. decrease the volume.
c. increase the pressure.
d. lower the temperature.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1


Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3
You know the right answer?
H2 and i2 react in an exothermic reaction according to the following equation:
h2(g) + i2(g)...
h2(g) + i2(g)...
Questions

Mathematics, 17.10.2019 01:20

Geography, 17.10.2019 01:20




Mathematics, 17.10.2019 01:20



History, 17.10.2019 01:20


Mathematics, 17.10.2019 01:20

Mathematics, 17.10.2019 01:20


History, 17.10.2019 01:20

Health, 17.10.2019 01:20

Mathematics, 17.10.2019 01:20


History, 17.10.2019 01:20

History, 17.10.2019 01:20