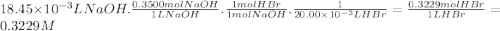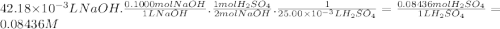Chemistry, 13.11.2019 21:31 ieshaking28
Stu dent has finished his titration, and he comes to you for with the calculations. he tells you that 20.00 ml of unknown concentration hbr(aq) required 18.45 ml of 0.3500 m naoh(aq) to neutralize it, to the point where thymol blue indicator changed from pale yellow to very pale blue. calculate the concentration (molarity) of stu's hbr(aq) sample. kemmi major also does a titration. she measures 25.00 ml of unknown concentration h_2so_4(aq) and titrates it with 0.1000 m naoh(aq). when she has added 42.18 ml of the base, her phenolphthalein indicator turns light pink. what is the concentration (molarity) of kemmi's h_2so_4(aq) sample?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Theoretically, which metal should be the most reactive? hydrogen lithium francium fluorine
Answers: 1

Chemistry, 21.06.2019 17:00
What is the nature of the ca-cl bond in a molecule of calcium chloride (cacl2) if the electronegativity value of calcium is 1.0 and that of chlorine is 3.16?
Answers: 1

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3
You know the right answer?
Stu dent has finished his titration, and he comes to you for with the calculations. he tells you th...
Questions

Mathematics, 27.06.2020 22:01

Social Studies, 27.06.2020 22:01

Biology, 27.06.2020 22:01

Mathematics, 27.06.2020 22:01

English, 27.06.2020 22:01

Mathematics, 27.06.2020 22:01

History, 27.06.2020 22:01


Biology, 27.06.2020 22:01


Mathematics, 27.06.2020 22:01


History, 27.06.2020 22:01


Mathematics, 27.06.2020 22:01

Mathematics, 27.06.2020 22:01

World Languages, 27.06.2020 22:01

Physics, 27.06.2020 22:01