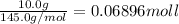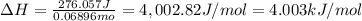Chemistry, 13.11.2019 19:31 danbat3023
Acoffee cup calorimeter was used to measure the heat of solution, the change in enthalpy that occurs when a solid dissolves in water. a 10.0 g sample of an ionic compound with a molar mass of 145.0 g/mol was added to a sample of deionized water to produce 60.0 grams of solution. after stirring and dissolving the solid, the temperature was found to change from 25.00 ∘c to 23.89 ∘c . calculate the enthalpy of solution, δhsoln , per mole of salt dissolved. assume the specific heat of the solution is 4.06 j/(g⋅∘c ) and the heat capacity of the calorimeter is 5.10 j/ ∘c . calculate the heat change experienced by the calorimeter contents, . = j calculate the heat change experienced by the calorimeter, . = j calculate the heat change produced by the solution process, . = j calculate δhsoln , the enthalpy of solution for one mole of solid in kilojoules per mole. δhsoln= kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2
You know the right answer?
Acoffee cup calorimeter was used to measure the heat of solution, the change in enthalpy that occurs...
Questions

Advanced Placement (AP), 04.02.2020 08:58


Mathematics, 04.02.2020 08:58




History, 04.02.2020 08:58

Physics, 04.02.2020 08:58

Mathematics, 04.02.2020 08:58





Mathematics, 04.02.2020 08:58

Mathematics, 04.02.2020 08:58

Mathematics, 04.02.2020 08:58

Biology, 04.02.2020 08:58

Social Studies, 04.02.2020 08:58


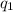
 = change in temperature = -1.11°C
= change in temperature = -1.11°C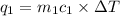
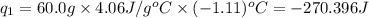
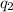
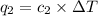
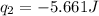
![Q=[-q_1+(-q_2)]](/tpl/images/0372/7156/e1e62.png)
![Q=[-(m_1c_1\times \Delta T)+(-c_2\times \Delta T)]](/tpl/images/0372/7156/ff7dd.png)
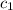 = specific heat of solution =
= specific heat of solution = 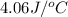
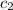 = specific heat of calorimeter=
= specific heat of calorimeter= 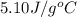
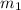 = mass of solution= 60.0 g
= mass of solution= 60.0 g![Q=[-(60.0 g\times 4.06J/g^oC\times (-1.11)^oC)+(- 5.10J/^oC\times (-1.11)^oC)]](/tpl/images/0372/7156/5fbbc.png)
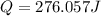
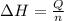
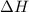 = enthalpy change = ?
= enthalpy change = ?