
Chemistry, 13.11.2019 06:31 PrincessKehlani15
The oxidation of glucose can result in several products. you perform combustion analysis on a 2.750 g sample that resulted from the oxidation of glucose and obtain 3.702 g co2 and 1.514 g h2o. how many carbon atoms are in the empirical formula of the isolated sample? (assume no other products present from the oxidation of glucose.)]

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1
You know the right answer?
The oxidation of glucose can result in several products. you perform combustion analysis on a 2.750...
Questions


Mathematics, 06.05.2020 08:06

Mathematics, 06.05.2020 08:06

Biology, 06.05.2020 08:06



Social Studies, 06.05.2020 08:06



Mathematics, 06.05.2020 08:06

English, 06.05.2020 08:06


Mathematics, 06.05.2020 08:06


Mathematics, 06.05.2020 08:06


Mathematics, 06.05.2020 08:06



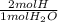 = 0.1682 mol H
= 0.1682 mol H

