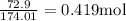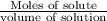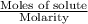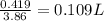Chemistry, 13.11.2019 06:31 nuconteaza119
How many liters of a 3.86 m k2so4 solution are needed to provide 72.9 g of k2so4 (molar mass 174.01 g/mol)? recall that m is equivalent to mol/l.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1



Chemistry, 23.06.2019 05:30
Based on the formulas, select the compounds below that are covalent: kbr sif4 al2o3 co2 naco3 s7o2 pcl3 fe3n2 h2o s2f10
Answers: 3
You know the right answer?
How many liters of a 3.86 m k2so4 solution are needed to provide 72.9 g of k2so4 (molar mass 174.01...
Questions




Mathematics, 05.02.2020 13:47


Biology, 05.02.2020 13:47

History, 05.02.2020 13:47

Mathematics, 05.02.2020 13:47


Biology, 05.02.2020 13:47


Mathematics, 05.02.2020 13:47







Arts, 05.02.2020 13:47

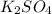 will be needed
will be needed