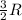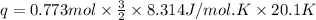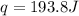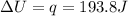A0.773 mol sample of xe(g) initially at 298 k and 1.00 atm is held at constant volume while enough heat is applied to raise the temperature of the gas by 20.1 k. assuming ideal gas behavior, calculate the amount of heat in joules (q) required to affect this temperature change and the total change in internal energy, ? u. note that some books use ? e as the symbol for internal energy instead of ? u.
q= ? u=

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2
You know the right answer?
A0.773 mol sample of xe(g) initially at 298 k and 1.00 atm is held at constant volume while enough h...
Questions





Biology, 27.06.2019 10:00



Mathematics, 27.06.2019 10:00


English, 27.06.2019 10:00




Computers and Technology, 27.06.2019 10:00





Biology, 27.06.2019 10:00

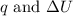 is 193.8 J and 193.8 J respectively.
is 193.8 J and 193.8 J respectively.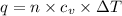
 = Change in temperature = 20.1 K
= Change in temperature = 20.1 K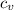 = heat capacity at constant volume of Xe (mono-atomic molecule) =
= heat capacity at constant volume of Xe (mono-atomic molecule) =