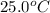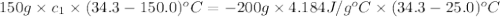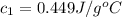Chemistry, 13.11.2019 03:31 champton79
Nunknown metal is either aluminum, iron or lead. if 150. g of this metal at 150.0 °c was placed in a calorimeter that contains 200. g of water at 25.0 °c and the final temperature was found to be 34.3 °c after thermal equilibrium was achieved, assuming heat was only transferred between water and metal, what is the identity of this metal? some specific heat values of metals and water given below may be useful. specific heats, j/(g•°c): fe (0.449) pb (0.128) al (0.903) h2o (4.184)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:40
Astudent made the lewis dot diagram of a compound shown. what is the error in the lewis dot diagram? a)an o atom should transfer all of its six electrons to mg because the formula is mgo b) both electrons of mg should be transferred to one o adam because the formula is mgo c) the electrons should be transferred from each o add him to capital mg because mg has fewer electrons d) the number of dots around mg should be four because it has to transfer two electrons to each o
Answers: 1

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3
You know the right answer?
Nunknown metal is either aluminum, iron or lead. if 150. g of this metal at 150.0 °c was placed in a...
Questions

Biology, 05.05.2021 18:50


Spanish, 05.05.2021 18:50

Mathematics, 05.05.2021 18:50





Mathematics, 05.05.2021 18:50

Chemistry, 05.05.2021 18:50


Mathematics, 05.05.2021 18:50

Mathematics, 05.05.2021 18:50





Health, 05.05.2021 18:50

Mathematics, 05.05.2021 18:50

Mathematics, 05.05.2021 18:50

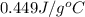 ).
).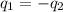
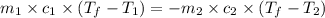
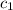 = specific heat of unknown metal = ?
= specific heat of unknown metal = ?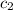 = specific heat of water =
= specific heat of water = 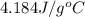
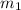 = mass of unknown metal = 150 g
= mass of unknown metal = 150 g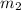 = mass of water = 200 g
= mass of water = 200 g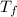 = final temperature of water =
= final temperature of water = 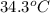
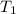 = initial temperature of unknown metal =
= initial temperature of unknown metal = 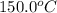
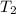 = initial temperature of water =
= initial temperature of water =