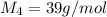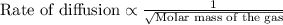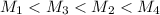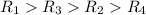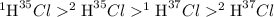Chemistry, 13.11.2019 02:31 shoafmckenzie1962
Hydrogen has two naturally occurring isotopes, 1h and 2h. chlorine also has two naturally occurring isotopes, 35cl and 37cl. thus, hydrogen chloride gas consists of four distinct types of molecules: 1h35cl, 1h37cl, 2h35cl, and 2h37cl. place these four molecules in order of decreasing rate of effusion.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 23.06.2019 06:00
What does it mean for something to be dissolved in watera- it is submerged in water moleculesb-it is stirred in the water moleculesc- it is surrounded by water molecules d-it has water molecules added to it
Answers: 2

Chemistry, 23.06.2019 09:40
Write balanced nuclear equations for the formation of five elements whose atomic number is between helium (2) and iron (26):
Answers: 1

Chemistry, 23.06.2019 11:30
Jenny places a strip of ph paper into a solution. when she removes the ph paper, it has turned yellow-green. what should jenny do next to determine the ph of her solution? a. use a different testing method because the ph paper should not change colors b. place the ph paper into a machine that reads the ph of the solution c. compare the ph paper's color with the color of ph paper from another solution d. compare the ph paper's color with a chart of colors and ph ranges
Answers: 1
You know the right answer?
Hydrogen has two naturally occurring isotopes, 1h and 2h. chlorine also has two naturally occurring...
Questions

Mathematics, 02.10.2020 14:01

History, 02.10.2020 14:01




Mathematics, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

Advanced Placement (AP), 02.10.2020 14:01


Mathematics, 02.10.2020 14:01

History, 02.10.2020 14:01





History, 02.10.2020 14:01




Social Studies, 02.10.2020 14:01

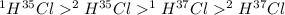
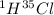 gas=
gas= 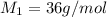
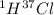 gas =
gas =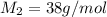
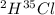 gas =
gas =
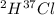 gas =
gas =