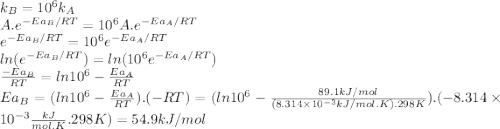Chemistry, 13.11.2019 02:31 joseperez1224
The standard free energy of activation of a reaction a is 81.9 kj mol–1 (19.6 kcal mol–1) at 298 k. reaction b is one million times faster than reaction a at the same temperature. the products of each reaction are 10.0 kj mol–1 (2.39 kcal mol–1) more stable than the reactants. (a) what is the standard free energy of activation of reaction b?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3
You know the right answer?
The standard free energy of activation of a reaction a is 81.9 kj mol–1 (19.6 kcal mol–1) at 298 k....
Questions

Mathematics, 25.04.2020 07:17


Biology, 25.04.2020 07:17

English, 25.04.2020 07:17

Business, 25.04.2020 07:18


Mathematics, 25.04.2020 07:18

Mathematics, 25.04.2020 07:18


English, 25.04.2020 07:18

Chemistry, 25.04.2020 07:18

English, 25.04.2020 07:18

History, 25.04.2020 07:18

Mathematics, 25.04.2020 07:18


Mathematics, 25.04.2020 07:18




Mathematics, 25.04.2020 07:18

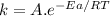
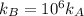 .
.