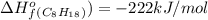Chemistry, 13.11.2019 00:31 ridzrana02
For a particular isomer of c8h18,c8h18, the combustion reaction produces 5099.5 kj 5099.5 kj of heat per mole of c8h18(g)c8h18(g) consumed, under standard conditions. c8h18(g)+252o2(g)⟶8co2(g)+9h2o(g)δh ∘rxn=−5099.5 kj/mol c8h18(g)+252o2(g)⟶8co2(g)+9h2o(g)δh rxn°=−5099.5 kj/mol what is the standard enthalpy of formation of this isomer of c8h18(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1

Chemistry, 23.06.2019 01:00
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3
You know the right answer?
For a particular isomer of c8h18,c8h18, the combustion reaction produces 5099.5 kj 5099.5 kj of heat...
Questions

Chemistry, 08.07.2020 05:01


Mathematics, 08.07.2020 05:01





Health, 08.07.2020 05:01



Mathematics, 08.07.2020 05:01


Health, 08.07.2020 05:01

Mathematics, 08.07.2020 05:01

Chemistry, 08.07.2020 05:01

Mathematics, 08.07.2020 05:01

Mathematics, 08.07.2020 05:01



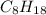 is -222 kJ/mol
is -222 kJ/mol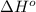
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0371/3800/45485.png)
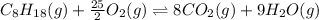
![\Delta H^o_{rxn}=[(n_{(CO_2)}\times \Delta H^o_f_{(CO_2)})+(n_{(H_2O)}\times \Delta H^o_f_{(H_2O)})]-[(n_{(C_8H_{18})}\times \Delta H^o_f_{(C_8H_{18})})+(n_{(O_2)}\times \Delta H^o_f_{(O_2)})]](/tpl/images/0371/3800/70c63.png)
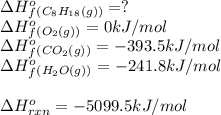
![-5099.5=[(8\times -393.5)+(9\times -241.5)]-[(1\times \Delta H^o_f_{(C_8H_{18})}))+(\frac{25}{2}\times 0)]](/tpl/images/0371/3800/1a9fc.png)