
Chemistry, 12.11.2019 23:31 maskoffvon
How many 3-liter balloons could the 12-l helium tank pressurized to 160 atm fill? keep in mind that an "exhausted" helium tank is not empty. in other words, once the gas inside the tank reaches atmospheric pressure, it will no longer be able to fill balloons. express the number of balloons to three significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2
You know the right answer?
How many 3-liter balloons could the 12-l helium tank pressurized to 160 atm fill? keep in mind that...
Questions




English, 13.10.2019 20:50

Geography, 13.10.2019 20:50

English, 13.10.2019 20:50


History, 13.10.2019 20:50

Physics, 13.10.2019 20:50

Mathematics, 13.10.2019 20:50


Mathematics, 13.10.2019 20:50


Mathematics, 13.10.2019 20:50


History, 13.10.2019 20:50



Mathematics, 13.10.2019 20:50

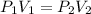
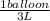 = 636 balloons
= 636 balloons

