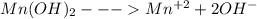Chemistry, 12.11.2019 23:31 chassidytjtrimb
Compare the solubility of mn(oh)2 (ksp = 1.6x10-13)in the following solutions: pure water, a solution buffered to be ph = 6.5 a solution buffered to be ph = 3.5 compare the solubility of mn(oh)2 (ksp = 1.6x10-13)in the following solutions: pure water, a solution buffered to be ph = 6.5 a solution buffered to be ph = 3.5 pure water > ph 3.5 > ph 6.5 (least soluble) ph 3.5 > ph 6.5 > pure water (least soluble) ph 3.5 > pure water > ph 6.5 (least soluble) pure water > ph 6.5 > ph 3.5 (least soluble) ph 6.5 > ph 3.5 > pure water (least soluble)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1


Chemistry, 23.06.2019 11:30
All of the following describe uses of nonrenewable energy sources except
Answers: 3

Chemistry, 23.06.2019 13:00
Write the balanced chemical reaction for the formation of fe2(so4)3 from fe2o3 and so3 and determine how many moles of fe2(so4)3 are formed when 12.7 mol of so3 are reacted.
Answers: 1
You know the right answer?
Compare the solubility of mn(oh)2 (ksp = 1.6x10-13)in the following solutions: pure water, a soluti...
Questions

Mathematics, 20.07.2019 18:00



Mathematics, 20.07.2019 18:00


Physics, 20.07.2019 18:00



Mathematics, 20.07.2019 18:00

SAT, 20.07.2019 18:00


Mathematics, 20.07.2019 18:00






Chemistry, 20.07.2019 18:00