
Chemistry, 12.11.2019 04:31 CameronVand21
Hardness in groundwater is due to the presence of metal ions, primarily mg2+ and ca2+ . hardness is generally reported as ppm caco3 . to measure water hardness, a sample of groundwater is titrated with edta, a chelating agent, in the presence of the indicator eriochrome black t, symbolized as in. eriochrome black t, a weaker chelating agent than edta, is red in the presence of ca2+ and turns blue when ca2+ is removed. red blue ca(in)2+ + edta ⟶ ca(edta)2+ + in a 50.00 ml sample of groundwater is titrated with 0.0450 m edta . if 13.70 ml of edta is required to titrate the 50.00 ml sample, what is the hardness of the groundwater in molarity and in parts per million of caco3 by mass? assume that ca2+ accounts for all of the hardness in the groundwater.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
If 200.0g of copper(ll) sulfate react with an excess of zinc metal, what is the theoretical yield of copper
Answers: 1

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1
You know the right answer?
Hardness in groundwater is due to the presence of metal ions, primarily mg2+ and ca2+ . hardness is...
Questions


Mathematics, 26.10.2019 20:43

English, 26.10.2019 20:43






Mathematics, 26.10.2019 20:43

Mathematics, 26.10.2019 20:43


English, 26.10.2019 20:43


Mathematics, 26.10.2019 20:43

Biology, 26.10.2019 20:43

History, 26.10.2019 20:43

English, 26.10.2019 20:43

History, 26.10.2019 20:43

Business, 26.10.2019 20:43

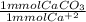 * 100.09 mg/mmol = 61.70 mg CaCO₃
* 100.09 mg/mmol = 61.70 mg CaCO₃

