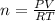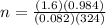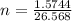Chemistry, 12.11.2019 04:31 ChloeLiz7111
Consider the chemical reaction:
2h2o(l)→2h2(g)+o2(g)
how many moles of h2o are required to form 1.6 l of o2 at a temperature of 324 k and a pressure of 0.984 atm ?
express your answer using two significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
You know the right answer?
Consider the chemical reaction:
2h2o(l)→2h2(g)+o2(g)
how many moles of h2o are required...
2h2o(l)→2h2(g)+o2(g)
how many moles of h2o are required...
Questions

Biology, 27.11.2019 11:31

Mathematics, 27.11.2019 11:31



Biology, 27.11.2019 11:31


History, 27.11.2019 11:31



Mathematics, 27.11.2019 11:31

Mathematics, 27.11.2019 11:31



Mathematics, 27.11.2019 11:31

English, 27.11.2019 11:31

Mathematics, 27.11.2019 11:31


Physics, 27.11.2019 11:31

Mathematics, 27.11.2019 11:31

Mathematics, 27.11.2019 11:31