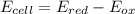Chemistry, 11.11.2019 23:31 mariamakonteh31
In the activity, click on the e∘cell and keq quantities to observe how they are related. use this relation to calculate keq for the following redox reaction that occurs in an electrochemical cell having two electrodes: a cathode and an anode. the two half-reactions that occur in the cell are
cu2+(aq)+2e−→cu(s) and co(s)→co2+(aq)+2e−
the net reaction is
cu2+(aq)+co(s)→cu(s)+co2+(aq)
use the given standard reduction potentials in your calculation as appropriate. ( keq=5.88*10^20)
in the activity, click on the keq and δg∘ quantities to observe how they are related.
calculate δg∘ using this relationship and the equilibrium constant (keq) obtained in part a at t=298k: keq=5.88*10^20
express the gibbs free energy (δg∘) in joules to three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2


Chemistry, 23.06.2019 13:50
Which compounds are the brønsted-lowry bases in this equilibrium: hc2o4– + h2bo3– h3bo3 + c2o42– ? a. h2bo3– and h3bo3 b. hc2o4– and h3bo3 c. hc2o4– and c2o42– d. h2bo3– and c2o42– e. hc2o4– and h2bo3–
Answers: 2
You know the right answer?
In the activity, click on the e∘cell and keq quantities to observe how they are related. use this re...
Questions


Physics, 15.12.2020 18:30

Mathematics, 15.12.2020 18:30







Mathematics, 15.12.2020 18:30






Mathematics, 15.12.2020 18:30

English, 15.12.2020 18:30


Mathematics, 15.12.2020 18:30