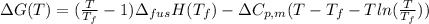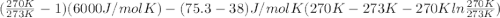Chemistry, 11.11.2019 23:31 okayfine3411
Calculate the gibbs energy of freezing (deltagfreezing) in units of j/mol when supercooled water freezes at -3degreec at constant t and p. delta h fusion = 6000 j/mol at 0degreec. the molar heat capacity of water and ice are 75.3 j/molk and 38 j/molk, respectively, and both are independent of temperature over this range. state any assumptions you make in your calculation!

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1
You know the right answer?
Calculate the gibbs energy of freezing (deltagfreezing) in units of j/mol when supercooled water fre...
Questions

Biology, 20.10.2020 23:01



English, 20.10.2020 23:01


English, 20.10.2020 23:01

Mathematics, 20.10.2020 23:01

Mathematics, 20.10.2020 23:01


Mathematics, 20.10.2020 23:01

English, 20.10.2020 23:01

Chemistry, 20.10.2020 23:01

Mathematics, 20.10.2020 23:01

History, 20.10.2020 23:01

English, 20.10.2020 23:01

Business, 20.10.2020 23:01


Mathematics, 20.10.2020 23:01


Spanish, 20.10.2020 23:01

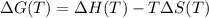
![\Delta H(T_{f}) - \Delta C_{p,m} (T - T_{f}) - T[\Delta S(T_{f}) - \Delta C_{p,m} ln (\frac{T}{T_{f}})]](/tpl/images/0369/6715/c43d6.png)
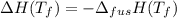 (assumption)
(assumption)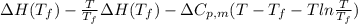
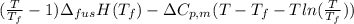
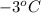 = (-3 + 273) = 270 K,
= (-3 + 273) = 270 K, 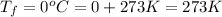 .
.