Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
You know the right answer?
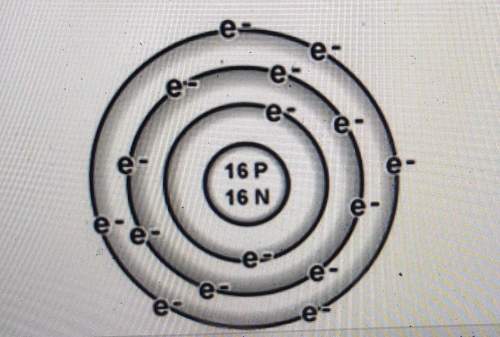
Is the atom below more likely to gain electrons or to lose electrons? explain how you can tell.
Questions

Mathematics, 02.02.2021 18:20

Mathematics, 02.02.2021 18:20

History, 02.02.2021 18:20




Chemistry, 02.02.2021 18:20

Mathematics, 02.02.2021 18:20


Mathematics, 02.02.2021 18:20




French, 02.02.2021 18:20


Mathematics, 02.02.2021 18:20

Physics, 02.02.2021 18:20



History, 02.02.2021 18:20