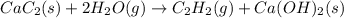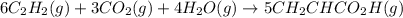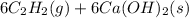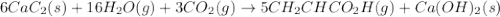There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide:
cac2(s)+2h2o(g)→c2h2(g)+caoh2(s)
in the second step, acetylene, carbon dioxide and water react to form acrylic acid:
6c2h2(g)+3co2(g)+4h2o(g)→5ch2chco2h (g)
write the net chemical equation for the production of acrylic acid from calcium carbide, water and carbon dioxide. be sure your equation is balanced.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2
You know the right answer?
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of...
Questions

English, 23.06.2020 10:57

English, 23.06.2020 10:57

Mathematics, 23.06.2020 10:57

Biology, 23.06.2020 10:57

English, 23.06.2020 10:57








Mathematics, 23.06.2020 10:57


Business, 23.06.2020 10:57

English, 23.06.2020 10:57

Mathematics, 23.06.2020 10:57

Chemistry, 23.06.2020 10:57

History, 23.06.2020 10:57