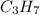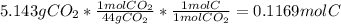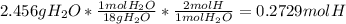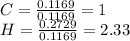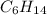A1.678 g sample of a component of the light petroleum distillate called naphtha is found to yield 5.143 g co2 (g) and 2.456 g h2o (l) on complete combustion. this particular compound is also found to be an alkane with one methyl group attached to a longer carbon chain and to have a molecular formula twice its empirical formula. the compound also has the following properties: melting point of -154 c , boiling point of 60.3 c , density of 0.6532 g/ml at 20 c , specific heat of 2.25 j/(g*c), and -204.6 kj/mol use the masses of carbon dioxide, co2, and water, h2o , to determine the empirical formula of the alkane component. express your answer as a chemical formula?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

You know the right answer?
A1.678 g sample of a component of the light petroleum distillate called naphtha is found to yield 5....
Questions

Mathematics, 23.04.2020 18:28


Biology, 23.04.2020 18:28

Geography, 23.04.2020 18:28




Mathematics, 23.04.2020 18:28

History, 23.04.2020 18:28

Arts, 23.04.2020 18:28


Mathematics, 23.04.2020 18:28






Mathematics, 23.04.2020 18:28

Biology, 23.04.2020 18:28

Mathematics, 23.04.2020 18:28