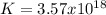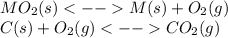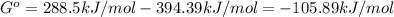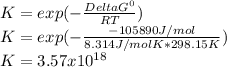Chemistry, 10.11.2019 07:31 lovelyheart5337
When the oxide of generic metal m is heated at 25c, only a negligible amount of m is produced. mo2(s) < > m(s)+o2(g) delta g = 288.5 kj/mol
1.) when the reaction is coupled to the conversion of graphite to carbon dioxide, it becomes spontaneous. what is the chemical equation of this coupled process? show that the reaction is in equilibrium, include physical states, and represent graphite as c(s)
2.) what is the thermodynamic equilibrium constant for the coupled reaction? k =

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1

Chemistry, 23.06.2019 06:00
What physical property of gold makes panning a useful way to get gold from streams?
Answers: 2
You know the right answer?
When the oxide of generic metal m is heated at 25c, only a negligible amount of m is produced. mo2(s...
Questions

English, 04.11.2020 06:50


History, 04.11.2020 06:50

Mathematics, 04.11.2020 06:50

Computers and Technology, 04.11.2020 06:50

History, 04.11.2020 06:50


Computers and Technology, 04.11.2020 06:50

Social Studies, 04.11.2020 06:50


Mathematics, 04.11.2020 06:50


Mathematics, 04.11.2020 06:50

Arts, 04.11.2020 06:50


Mathematics, 04.11.2020 06:50

Spanish, 04.11.2020 06:50

Business, 04.11.2020 06:50

French, 04.11.2020 06:50

English, 04.11.2020 06:50