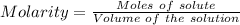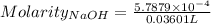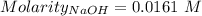Chemistry, 10.11.2019 06:31 maddietomlinson113
Ask your teacher sodium hydroxide solution is usually standardized by titrating a pure sample of potassium hydrogen phthalate (khc8h4o4, often abbreviated khp), an acid with one acidic hydrogen and a molar mass of 204.220 g/mol. it takes 36.01 ml of a sodium hydroxide solution to titrate a 0.1182-g sample of khp. what is the molarity of the sodium hydroxide

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1

Chemistry, 23.06.2019 06:30
The polarity of an oxygen-hydrogen bond is higher than the polarity of a nitrogen-hydrogen bond, allowing amines to be more soluble than alcohols.
Answers: 3
You know the right answer?
Ask your teacher sodium hydroxide solution is usually standardized by titrating a pure sample of pot...
Questions



Biology, 17.12.2019 13:31



Physics, 17.12.2019 13:31





Business, 17.12.2019 13:31

History, 17.12.2019 13:31


English, 17.12.2019 13:31

Mathematics, 17.12.2019 13:31



History, 17.12.2019 13:31


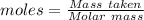
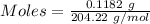
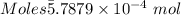
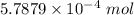 of KHP reacts with
of KHP reacts with