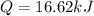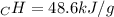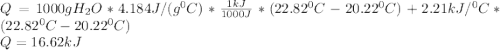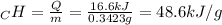Chemistry, 10.11.2019 06:31 angelica9613
A0.3423 g sample of pentane, c5h12, was burned in a bomb calorimeter. the temperature of the calorimeter and the 1.000kg of water contained therein rose from 20.22 degrees celcius to 22.82 degrees celcius. the heat capacity of the calorimeter is 2.21 kj/c. the heat capacity of water = 4.184 j/g c. a. how much heat was given off during combustion fo the sample of pentane. answer = 16.6 kjb. what is the heat of combustion, in kilojoules, per gram of pentaneanswer = 48.6 kj/g

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3


Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2
You know the right answer?
A0.3423 g sample of pentane, c5h12, was burned in a bomb calorimeter. the temperature of the calorim...
Questions

Mathematics, 10.04.2021 01:00

Mathematics, 10.04.2021 01:00


Mathematics, 10.04.2021 01:00

Chemistry, 10.04.2021 01:00

Biology, 10.04.2021 01:00


Mathematics, 10.04.2021 01:00


English, 10.04.2021 01:00

Mathematics, 10.04.2021 01:00


Mathematics, 10.04.2021 01:00


English, 10.04.2021 01:00



Mathematics, 10.04.2021 01:00


History, 10.04.2021 01:00