
Chemistry, 10.11.2019 05:31 battlemarshmell
Aqueous sulfuric acid reacts with solid sodium hydroxide to produce aqueous sodium sulfate and liquid water . if of sodium sulfate is produced from the reaction of of sulfuric acid and of sodium hydroxide, calculate the percent yield of sodium sulfate. be sure your answer has the correct number of significant digits in it.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
You know the right answer?
Aqueous sulfuric acid reacts with solid sodium hydroxide to produce aqueous sodium sulfate and liqui...
Questions

Health, 21.07.2019 14:00

Health, 21.07.2019 14:00


History, 21.07.2019 14:00


Biology, 21.07.2019 14:00

Social Studies, 21.07.2019 14:00


Social Studies, 21.07.2019 14:00




Mathematics, 21.07.2019 14:00



History, 21.07.2019 14:00





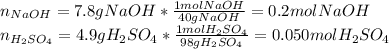
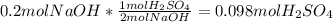
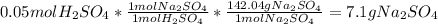
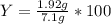
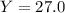 %
%

