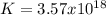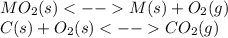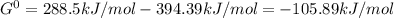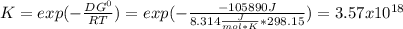Chemistry, 10.11.2019 04:31 shadowsnake
When the oxide of generic metal m is heated at 25c, only a negligible amount of m is produced. mo2(s) < > m(s)+o2(g)delta g = 291.0 kj/mol1.) when the reaction is coupled to the conversion of graphite to carbon dioxide, it becomes spontaneous. what is the chemical equation of this coupled process? show that the reaction is in equilibrium, include physical states, and represent graphite as c(s) .) what is the thermodynamic equilibrium constant for the coupled reaction? k =

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Strong conductivity of plasma allows it to act and react as and
Answers: 2

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2
You know the right answer?
When the oxide of generic metal m is heated at 25c, only a negligible amount of m is produced. mo2(s...
Questions

Mathematics, 28.06.2019 17:00

Social Studies, 28.06.2019 17:00


History, 28.06.2019 17:00

Geography, 28.06.2019 17:00


Spanish, 28.06.2019 17:00

Mathematics, 28.06.2019 17:00

Social Studies, 28.06.2019 17:00


Social Studies, 28.06.2019 17:00

Biology, 28.06.2019 17:00




English, 28.06.2019 17:00




English, 28.06.2019 17:00