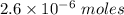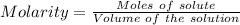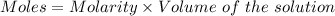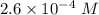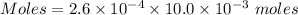Chemistry, 10.11.2019 03:31 shaylakabler333
Watch the video that shows using the dilution method to make a solution of known concentration. note that while the video shows the use of a beaker, in the lab we typically use a volumetric flask to make the diluted solution. the stock solution of kmno4 has a concentration of 2.6 × 10-4 m. the pipette has a volume of 10.0 ml. what is the amount of kmno4 delivered to the solution, in moles

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3


Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1
You know the right answer?
Watch the video that shows using the dilution method to make a solution of known concentration. note...
Questions


Mathematics, 11.02.2021 17:00

Mathematics, 11.02.2021 17:00

Mathematics, 11.02.2021 17:00



Physics, 11.02.2021 17:00


Mathematics, 11.02.2021 17:00



Mathematics, 11.02.2021 17:00

Mathematics, 11.02.2021 17:00

English, 11.02.2021 17:00



Chemistry, 11.02.2021 17:00

Mathematics, 11.02.2021 17:00