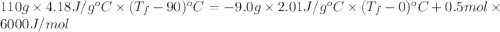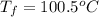Chemistry, 10.11.2019 03:31 julliette27
An ice cube of mass 9.0 g at temperature 0∘c is added to a cup of coffee, whose temperature is 90 ∘c and which contains 110 g of liquid. assume the specific heat capacity of the coffee is the same as that of water. the heat of fusion of ice (the heat associated with ice melting) is 6.0 kj/mol.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 23.06.2019 06:00
Give one example of a pure (exact) number and of an estimated (measured) number.
Answers: 2

Chemistry, 23.06.2019 10:00
What is the density, d, of a substance with a volume of v = 12.5 cm3 and a mass of m = 74.4 g ?
Answers: 1
You know the right answer?
An ice cube of mass 9.0 g at temperature 0∘c is added to a cup of coffee, whose temperature is 90 ∘c...
Questions



English, 26.05.2021 01:40


Advanced Placement (AP), 26.05.2021 01:40

Mathematics, 26.05.2021 01:40

English, 26.05.2021 01:40

Mathematics, 26.05.2021 01:40

Mathematics, 26.05.2021 01:40


Mathematics, 26.05.2021 01:40




Mathematics, 26.05.2021 01:40

Mathematics, 26.05.2021 01:40

History, 26.05.2021 01:40


History, 26.05.2021 01:40

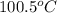
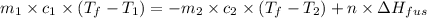
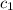 = specific heat of coffee = specific heat of water =
= specific heat of coffee = specific heat of water = 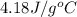 (as per question)
(as per question)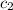 = specific heat of ice =
= specific heat of ice = 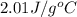
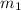 = mass of coffee = 110 g
= mass of coffee = 110 g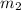 = mass of ice = 9.0 g
= mass of ice = 9.0 g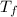 = final temperature = ?
= final temperature = ?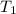 = initial temperature of coffee =
= initial temperature of coffee = 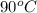
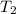 = initial temperature of ice =
= initial temperature of ice = 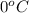
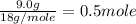
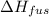 = 6.0 kJ/mol = 6000 J/mol
= 6.0 kJ/mol = 6000 J/mol