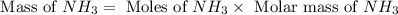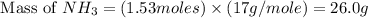Chemistry, 10.11.2019 01:31 maddiehope6140
Consider the following reaction: 2 no(g) + 5 h2(g) → 2 nh3(g) + 2 h2o(g) which set of solution maps would be needed to calculate the maximum amount of ammonia (nh3), in grams, that can be synthesized from 45.8 g of nitrogen monoxide (no) and 12.4 g of hydrogen (h2)? i. g no → mol no → mol nh3 → g nh3 ii. g h2 → mol h2 → mol nh3 → g nh3 iii. g no → mol no → mol h2o → g h2o iv. g h2 → mol h2 → mol h2o → g h2o

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Chemistry, 23.06.2019 02:30
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1
You know the right answer?
Consider the following reaction: 2 no(g) + 5 h2(g) → 2 nh3(g) + 2 h2o(g) which set of solution maps...
Questions









Mathematics, 19.07.2019 19:20

Computers and Technology, 19.07.2019 19:20

Computers and Technology, 19.07.2019 19:20



Computers and Technology, 19.07.2019 19:20

Computers and Technology, 19.07.2019 19:20





Computers and Technology, 19.07.2019 19:20

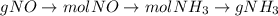
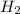 = 12.4 g
= 12.4 g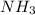 = 17 g/mole
= 17 g/mole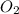 .
.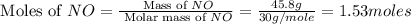

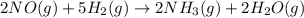
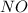 react with 5 mole of
react with 5 mole of 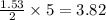 moles of
moles of