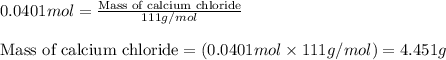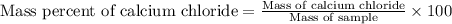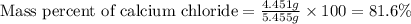Chemistry, 09.11.2019 08:31 Caixiayang3613
1. a 5.455-g sample of impure cacl2 is dissolved and treated with excess potassium carbonate solution. the dried caco3 (calcium carbonate) precipitate weighs 4.010-g. calculate the percent by mass of cacl2 in the original mixture.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 23.06.2019 02:30
Apound is approximately 0.45 kilogram. a persons weighs 87 kilograms. what is the persons’s weight, in pounds, when expressed to the correct number of significant figures
Answers: 1

Chemistry, 23.06.2019 07:00
4. glenn andrews recently bought a motorcycle for $3,950. if he had to pay 6% sales tax on the bike, what was the total cost of the motorcycle?
Answers: 1
You know the right answer?
1. a 5.455-g sample of impure cacl2 is dissolved and treated with excess potassium carbonate solutio...
Questions


Computers and Technology, 02.09.2020 21:01

History, 02.09.2020 21:01


Mathematics, 02.09.2020 21:01










Physics, 02.09.2020 21:01


Mathematics, 02.09.2020 21:01

Mathematics, 02.09.2020 21:01


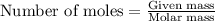 .....(1)
.....(1)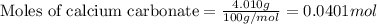
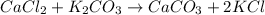
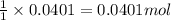 of calcium chloride
of calcium chloride