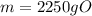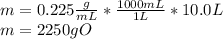Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3
You know the right answer?
Acertain organic compound o has a solubility in hexena of 0.225 at 10. °c. calculate the greatest ma...
Questions

Mathematics, 06.10.2020 06:01

Chemistry, 06.10.2020 06:01


English, 06.10.2020 06:01

Biology, 06.10.2020 06:01

Mathematics, 06.10.2020 06:01



Mathematics, 06.10.2020 06:01






Mathematics, 06.10.2020 06:01

Business, 06.10.2020 06:01

English, 06.10.2020 06:01

Mathematics, 06.10.2020 06:01