
Chemistry, 09.11.2019 05:31 brooklynpage3930
The heats of combustion of ethane (c2h6) and butane (c4h10) are 52 kj/g and 49 kj/g, respectively. we need to produce 1.000 x 103 kj heat by burning one of the fuels. which fuel will emit the least amount of co2? 1. calculate the number of grams needed of each fuel: 2. calculate the number of moles of each fuel: 3. write down the balanced chemical equation for the combustion of the fuels: 4. calculate the number of moles of co2 produced by burning each fuel to produce 1.000 x 103 kj. which fuel will emit the least amount of co2?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 23.06.2019 03:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 23.06.2019 08:30
This has nothing to do with school. i wrote a poem to my crush, who i'm asking out soon. tell me if it's cheesy, or cute. "roses are red, violets are blue no love story sounds right if it doesn't include you. dance with me all night, gaze into my eyes i'll hand you my heart, as well as my pride. when i hear your name, my heart goes insane. your all that i want, all that i need promise me you'll stay with me. here it is the final line, jasmine hill will you be mine? " i'm also going to buy her flowers, teddy bear and some food lol. written by me, bre (:
Answers: 2

You know the right answer?
The heats of combustion of ethane (c2h6) and butane (c4h10) are 52 kj/g and 49 kj/g, respectively. w...
Questions

Biology, 12.10.2020 01:01


History, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01

History, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01


Engineering, 12.10.2020 01:01

Spanish, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01

History, 12.10.2020 01:01

English, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01

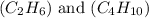 are 19.23 g and 20.41 g respectively.
are 19.23 g and 20.41 g respectively.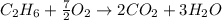
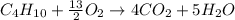
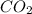 produced by burning each fuel is 1.28 mole and 1.41 mole respectively.
produced by burning each fuel is 1.28 mole and 1.41 mole respectively.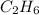
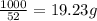
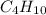 = 1 g
= 1 g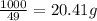
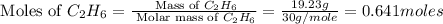
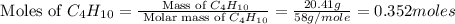
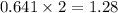 moles of
moles of 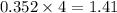 moles of
moles of 


